SOLIScript 1-step Multiplex Probe Kit
For sensitive and reliable quantification of RNA targets with probe based qPCR
Sino Biological launches the world largest recombinant viral antigen collection, ProVir™, which includes over 1000 products from 90 different types, 350 strains of viruses.
Recombinant antigens are key reagents in infectious disease research. They are widely used in the context of antibody and vaccine development. In addition, high-quality antigens and antibodies are critical components in immunodiagnostic assays.
Sino Biological Inc. launches the world’s largest recombinant viral antigen collection, ProVir™, which includes over 1000 products from 90 different types/subtypes and 350 strains of viruses. The product line features a wide range of high quality recombinant proteins expressed in insect and mammalian cells. These products are rigorously tested for their purity and bioactivity.
This unique portfolio includes the one-of-a-kind coronavirus catalog, a huge collection of infleunza antigens from over 250 strains, and many other hard-to-find viral proteins such as RSV, Ebola, and Cytomegalovirus. These reagents are specifically developed to help vaccine research and drug development.
Coronavirus are positive sense, single stranded RNA viruses. There are seven types of coronaviruses known to infect humans. Patients infected with these viruses develop respiratory symptoms of various severity. HCoV-229E and HCoV-OC43, the two coronaviruses discovered in early years, cause common cold. The other five coronaviruses lead to more severe respiratory tract infection, which can potentially be lethal. Since 2000, there have been three major world-wide health crisis caused by coronaviruses, the 2003 SARS outbreak, the 2012 MERS outbreak, and the 2019 COVID-19 outbreak. Thousands of people died during these epidemics, while surprisingly no vaccine, treatment, or diagnostic has been established. The outbreak of COVID-19 is yet another wake-up call for the biomedical community to make serious efforts to understand the biology of these viruses, and find ways to prevent and treat the infections.
| Types | Genera | Disease |
|---|---|---|
| SARS-CoV-2 | Betacoronavirus | Coronavirus disease 2019 (COVID-19). As of 14th July, >130M infected, >572K death. |
| SARS-CoV | Betacoronavirus | Severe acute respiratory syndrome (SARS), mortality rate 9% |
| MERS-CoV | Betacoronavirus | Middle East respiratory syndrome (MERS), mortality rate >30% |
| HCoV-HKU1 | Betacoronavirus | Mild respiratory disease |
| HCoV-NL63 | Alphacoronavirus | Mild respiratory disease |
| HCoV-OC43 | Betacoronavirus | Mild respiratory disease |
| HCoV-229E | Alphacoronavirus | Mild respiratory disease |
All coronaviruses share very similar structures. The viral genome encodes several proteins of unique functions, including Spike protein, N protein, HE protein, papain-like proteases, and M protein. The two antigens of main pharmaceutical interest are the S (spike) protein and the N protein. The N (nucleocapsid) protein is often conserved, which can be used as a diagnostic marker. The Spike protein is mainly responsible for receptor binding, and is a common target for vaccines and antibodies.
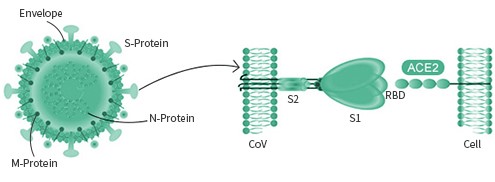
The spike protein is particular important as its interaction with the host cell receptor is the pivotal step during the infection. Different viruses may utilize different surface receptor for binding. The HCOV- NL63, SARS-COV, and the new SARS-COV-2 viruses all use the ACE2 receptor, while the MERS-COV virus selectively binds with the DPP4 receptor. The HCOV-229E virus targets APN receptor. The rest two common coronavirus, HKU1 and OC43 bind with O-ac Sia.
Influenza (flu) is a respiratory infection in mammals and birds. It is caused by an RNA virus in the family Orthomyxoviridae. Influenza virus is divided into four main types (Influenza A, Influenza B, Influenza C, Influenza D), which are distinguished by differences in two major internal proteins (hemagglutinin (HA) and neuraminidase (NA)). Three of the four types of influenza viruses affect humans: Type A, Type B, and Type C. Type D has not been known to infect humans, but is believed to have the potential to do so. Influenza virus type A is found in a wide variety of bird and mammal species and can undergo major shifts in immunological properties. Influenza virus type B is largely confined to humans and is an important cause of morbidity.
Little is known about Influenza virus type C, which is not an important source of morbidity. Influenza D was identified in 2016.
| Influenza A | Influenza B | Influenza C | |
|---|---|---|---|
| Hosts | Humans, waterfowl, poultry, pigs, horses, sea mammals, bats | Humans, seals | Humans, pigs, dogs |
| Gene segments | 8 | 8 | 7 |
| Proteins | 11 | 11 | 9 |
| HA/NA antigenic subtypes | 18 HA, 11 NA | None | None |
| Clinical features | Moderate to severe illness | Milder disease than Influenza A | Largely subclinical |
| Epidemiological features | Causes pandemics | Less severe epidemics than Influenza A; no pandemics | Does not cause epidemics or pandemics |
Influenza A virus is further divided into subtypes based on differences in the membrane proteins hemagglutinin (HA) and neuraminidase (NA), which are the most important targets for the immune system. The notation HhNn is used to refer to the subtype comprising the hth discovered Hemagglutinin (HA) protein and the nth discovered neuraminidase (NA) protein.
| H1 Subtype | H1N1 | H1N2 | H1N3 | H1N8 | H1N9 | |
| H2 Subtype | H2N2 | H2N3 | H2N8 | |||
| H3 Subtype | H3N1 | H3N2 | H3N8 | |||
| H4 Subtype | H4N2 | H4N4 | H4N6 | H4N8 | ||
| H5 Subtype | H5N1 | H5N2 | H5N3 | H5N6 | H5N8 | H5N9 |
| H6 Subtype | H6N1 | H6N2 | H6N4 | H6N5 | H6N6 | H6N8 |
| H7 Subtype | H7N1 | H7N2 | H7N3 | H7N7 | H7N8 | H7N9 |
| H8 Subtype | H8N4 | |||||
| H9 Subtype | H9N1 | H9N2 | H9N5 | H9N8 | ||
| H10 Subtype | H10N3 | H10N4 | H10N7 | H10N8 | H10N9 | |
| H11 Subtype | H11N2 | H11N6 | H11N9 | |||
| H12 Subtype | H12N1 | H12N3 | H12N5 | |||
| H13 Subtype | H13N6 | H13N8 | ||||
| H14 Subtype | H14N5 | |||||
| H15 Subtype | H15N2 | H15N8 | ||||
| H16 Subtype | H16N3 | |||||
| H17 Subtype | H17N10 | |||||
| H18 Subtype | H18N11 | |||||
| B influenza | Influenza B |
The influenza viral Hemagglutinin (HA) protein is a homo trimer with a receptor binding pocket on the globular head of each monomer, and the influenza viral neuraminidase (NA) protein is a tetramer with an enzyme active site on the head of each monomer. Subtypes are further divided into strains; each genetically distinct virus isolate is usually considered to be a separate strain.
We gladly support you by keeping you updated on our latest products and the developments around our services.